Shuangyang Building, Yangshe Town, Zhangjiagang City, Jiangsu Province, China.
Shuangyang Building, Yangshe Town, Zhangjiagang City, Jiangsu Province, China.
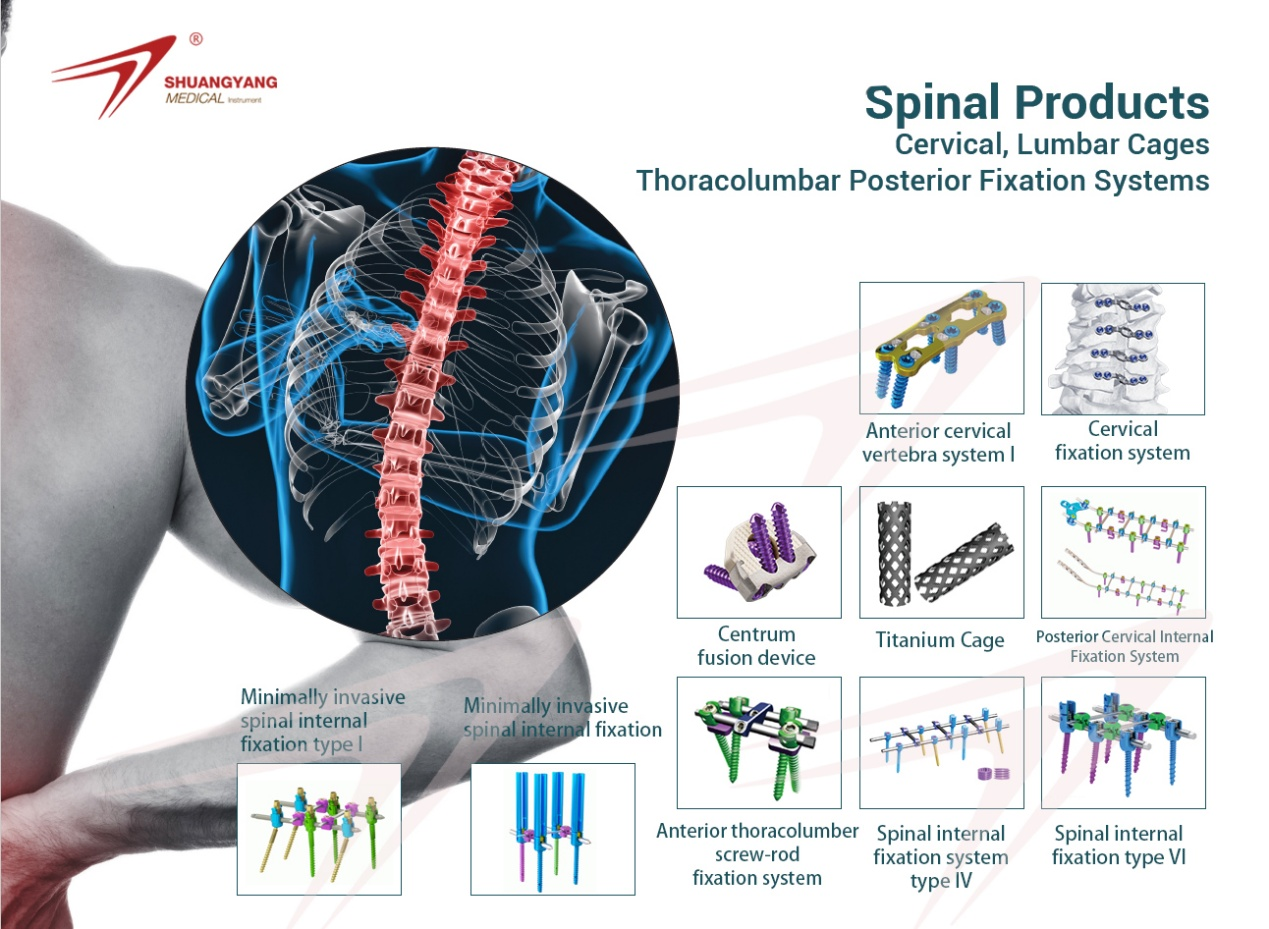
1.The pre-cut, pre-bending and diversified design of plate provide more clinical
options.
2.The plate surface adopt anodizing technology, can enhance surface hardness
and abrasive resistance.
3.The plate adopt sinking hole design, to make the plate and screw combine closer
to achieve low-profile fixation and reduce the discomfort of soft tissue.
4.The edges of plate are rounded to reduce irritation to the surrounding soft tissue.
Technical Specifications:
Product Name |
Item No. |
Specification |
|
Straight Plate Type I |
13.29.01.32006220 |
32mm*6holes |
13.29.01.64012220 |
64mm*12holes |
|
Straight Plate Type II |
13.29.01.55012221 |
55m*12holes |
|
Single bend plate |
13.29.03.25004220 |
25mm*4holes |
13.29.03.27004220 |
27mm*4holes |
|
13.29.03.29004220 |
29mm*4holes |
|
13.29.03.31004220 |
31mm*4holes |
|
13.29.03.33004220 |
33mm*4holes |
|
|
Double Bend Plate Type |
13.29.06.06004220 |
6mm*4holes |
13.29.06.07504220 |
7.5mm*4holes |
|
13.29.06.09004220 |
9mm*4holes |
|
13.29.06.10504220 |
10.5mm*4holes |
|
13.29.06.12004220 |
12mm*4holes |
|
|
Wide Mouth Plate Type |
13.29.07.08004221 |
8mm*4.8 |
13.29.07.10004221 |
10mm*4.8 |
|
13.29.07.11504221 |
11.5mm*4.8 |
|
13.29.07.08004222 |
8mm*6.8 |
|
13.29.07.10004222 |
10mm*6.8 |
|
13.29.07.11504222 |
11.5mm*6.8 |
|
|
Open Door Plate Type |
13.29.08.07504221 |
7.5mm*4.8 |
13.29.08.09504221 |
9.5mm*4.8 |
|
13.29.08.11504221 |
11.5mm*4.8 |
|
13.29.08.07504222 |
7.5mm*6.8 |
|
13.29.08.09504222 |
9.5mm*6.8 |
|
13.29.08.11504222 |
11.5mm*6.8 |
Conclusion
The laminoplasty fixation system, exemplified by titanium plates, fundamentally overcomes the uncertainties of traditional suspension techniques by providing rigid bony fixation. This approach achieves the comprehensive goals of controllable opening angles, sustained stability, and early patient mobilization, establishing it as the preferred and standard technique for posterior cervical spinal canal enlargement procedures.
A: Our products comply with international regulations and meet the necessary industry certifications. We are also certified to CE,ISO13485, ISO9001 and others. You can view more details of the certifications on the home page of our website.
A: shuangyang has a reliable shipping and logistics network to ensure prompt and secure delivery. Our team works closely with reputable carriers to provide efficient shipping services in around 120 countries.
A: The minimum order quantity may vary depending on the product and customizationrequirements, Pleasecontact our customer support team for specifc details related to your desired products.