Applying Core Lean Manufacturing Principles to Orthopedic Implant Assembly
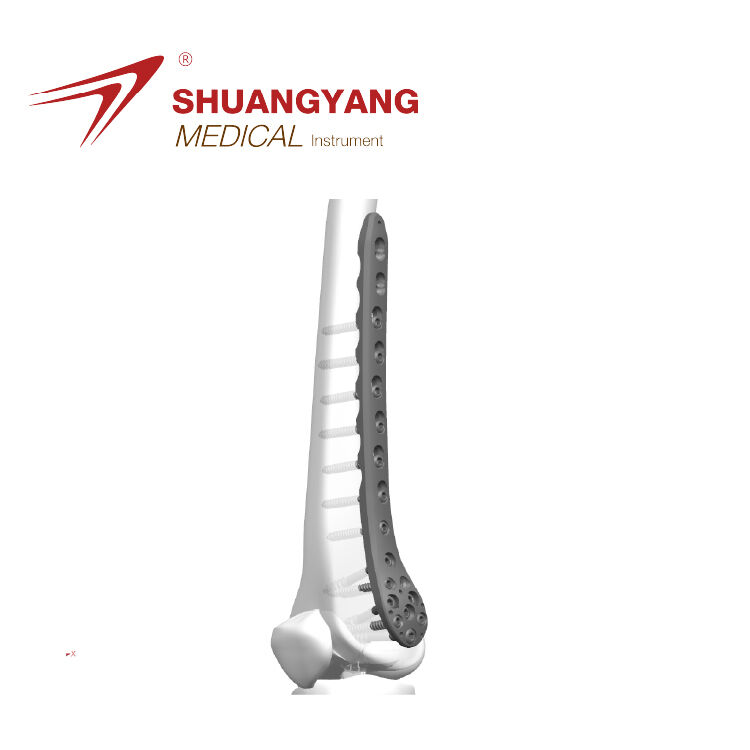
Mapping the Value Stream in Distal Humerus Plate Production
Value stream mapping, or VSM for short, helps manufacturers find places where they can cut down on waste during the assembly of those specialized distal humerus plates. When companies map out how materials move through their facilities—from when the raw titanium arrives all the way to when it gets packaged in sterile conditions—they start seeing where things get stuck. Common problems include doing the same calibration checks multiple times and having inspection stations that aren't properly aligned with production needs. According to research from the Ponemon Institute, nearly a third of all steps in these manufacturing processes don't actually add any real value, which translates into about $740,000 wasted each year on average. Today's successful VSM programs go beyond basic mapping by incorporating additional elements that make the whole system work better together.
- Digital twin simulations to model material handling and test layout changes virtually
- Real-time IoT tracking of milling tolerances to enable predictive quality control
- Cycle time analysis focused on critical path sequences—especially hole alignment and contouring
Identifying Non-Value-Added Steps in Sterile Packaging and Traceability Processes
When it comes to sterile packaging that meets ISO 13485 standards, there's actually quite a bit of hidden waste going on behind the scenes, even though everything looks compliant on paper. A lot of what happens isn't really adding value at all. Think about those tedious manual entries for UDI data, doing the same sterilization checks over and over again for basically identical product batches, plus all that extra work moving materials around during the blister sealing process. None of these things actually make products safer, more sterile, or better tracked in any meaningful way. And guess what? Industry players are starting to notice this waste and looking seriously at ways to cut it out. Automated systems for capturing data and smarter validation approaches based on actual risks rather than blanket requirements are becoming the go to solutions for many manufacturers trying to streamline operations without compromising quality.
Balancing FDA Validation Requirements with Lean Flow Continuity
The FDA validation process often gets in the way of smooth operations, but it doesn't have to be this way. Smart manufacturers are finding ways to balance strict regulations with efficient production methods. They use modular systems that focus on critical areas like final torque checks, while keeping other parts running smoothly. Some companies even run separate documentation teams alongside regular production shifts so nothing gets held up. And let's not forget about those predictive maintenance plans that keep machines running without unexpected breakdowns. These strategies actually work wonders when implemented properly. Many facilities report cutting down their validation cycles by around 35%, all while staying ready for audits and maintaining quality standards throughout the product release process.
Optimizing Process Efficiency in High-Precision Medical Device Assembly
Standardizing Work for Titanium Alloy Plate Fixation and Screw Hole Alignment
When it comes to making distal humerus plates, standardization really cuts down on all that variability by setting clear rules about where those screw holes go, how plates should be shaped, and what kind of torque needs applied with a tight ±0.2 Nm margin. The visual guides that come with these parts are pretty important too. They work alongside those geometric dimensioning specs and proper placement of fixtures to make sure everything lines up right, even when dealing with complicated polyaxial locking systems. According to last year's MedTech Quality Report, this method hits under 120 micrometers in positioning accuracy which is critical for successful bone fusion. Plus, manufacturers report cutting assembly mistakes almost in half at 47% reduction and bringing down problems during sterile packaging by around 32%. Makes sense why so many companies are adopting these standards now.
Reducing Cycle Time with Poka-Yoke Jigs in Final Torque Verification
Poka-yoke jigs help stop those pesky manual measurement mistakes when checking torque levels. These devices guide the alignment process using sensors and will actually alert workers if there's more than a half degree variation in angle during screw installation. When paired with digital torque drivers, these systems form what we call a closed loop feedback mechanism. This means adjustments happen right away instead of waiting for later checks. On average, validation time drops about 18 minutes per production batch, and companies report cutting down on wasted components by roughly 60%. What makes this approach so valuable? It keeps complete UDI traceability intact while ensuring titanium implants stay strong and reliable. Best part? Manufacturers don't have to add extra verification steps that would slow things down further.
Eliminating Waste in Lean Medical Device Manufacturing
Reducing Overprocessing in Multi-Stage Cleanroom Handling Under ISO 13485
Too much processing creates waste without making devices any safer or better performing. Think about all those extra cleanroom moves, repeated sterilization checks, and unnecessary inspections that just take time and money. Lean manufacturing methods can cut out these wasteful steps while still meeting ISO 13485 requirements. Take distal humerus plates for instance. Some companies have managed to reduce their sterile packaging steps by around a quarter through value stream mapping, and they haven't lost track of where everything goes. Pair this with clear step-by-step guides and error proofing systems, and manufacturers see faster production times without sacrificing quality control in their precision orthopedic parts. The industry is slowly realizing that doing less can actually lead to better results when done right.
Implementing Pull Systems and Lean Assembly Techniques
Kanban-Controlled Feeding of Anodized Screws and Polyaxial Locking Components
Kanban systems work by matching material flow to what's actually needed during surgeries through visual cues like colored bins or digital alerts instead of relying on old school batch processing based on forecasts. When applied to putting together orthopedic implants, these pull methods cut down on extra inventory for things like those anodized screws and those complicated polyaxial locking parts. Some facilities report cutting their excess stock around 40 percent while still keeping all the sterile packaging intact. The real game changer comes when automated Kanban signals connect directly with electronic health records and surgical schedules. This creates genuine just in time restocking scenarios that slash unnecessary handling, stop materials from expiring before they're used, and get implants ready faster without messing up tracking requirements or running afoul of regulations.
FAQ
What is Value Stream Mapping (VSM) and how does it apply to orthopedic implant assembly?
Value Stream Mapping is a tool that helps identify and eliminate waste in the manufacturing process. In orthopedic implant assembly, it helps identify inefficiencies from raw material arrival to finished product packaging.
How do Poka-Yoke jigs help in the manufacturing process?
Poka-Yoke jigs prevent manual measurement errors during torque verification, ensuring precise alignment and reducing waste.
What is the benefit of using Kanban systems in medical device assembly?
Kanban systems optimize material flow and inventory management, reducing excess stock and ensuring timely supply to match the demand during surgeries.
How does standardization impact the production of medical devices like distal humerus plates?
Standardization reduces variability, ensuring precise placement and alignment, which leads to increased accuracy and reduced assembly errors.
Table of Contents
- Applying Core Lean Manufacturing Principles to Orthopedic Implant Assembly
- Optimizing Process Efficiency in High-Precision Medical Device Assembly
- Eliminating Waste in Lean Medical Device Manufacturing
- Implementing Pull Systems and Lean Assembly Techniques
-
FAQ
- What is Value Stream Mapping (VSM) and how does it apply to orthopedic implant assembly?
- How do Poka-Yoke jigs help in the manufacturing process?
- What is the benefit of using Kanban systems in medical device assembly?
- How does standardization impact the production of medical devices like distal humerus plates?
 EN
EN
 FR
FR
 ES
ES
 AR
AR