Shuangyang Building, Yangshe Town, Zhangjiagang City, Jiangsu Province, China.
Shuangyang Building, Yangshe Town, Zhangjiagang City, Jiangsu Province, China.
Regulatory Divergence: EU MDR vs. FDA FrameworksClinical Evidence Requirements: Post-Market Surveillance vs. PMA PathwaysUnder the EU Medical Device Regulation, manufacturers must keep evaluating their products clinically throughout the entire life o...
VIEW MORE
Polymer-Based Antibiotic-Eluting Meshes: Mechanisms and Clinical ImpactHow Sustained-Release Coatings Enable Localized, Long-Term Antimicrobial ActivityAntibiotic releasing meshes made from polymers work by applying special coatings that slowly relea...
VIEW MORE
Leverage FDA Breakthrough Device Designation to Accelerate Regulatory Clearance Strategic Enrollment in the FDA Breakthrough Device Program for Rib Plating Systems The FDA's Breakthrough Device Program, commonly known as BDP, provides companies with...
VIEW MORE
Additive Manufacturing: Driving Customization and Biomechanical Optimization 3D-Printed Porous Titanium Cages for Enhanced Osseointegration and Anatomic Fit With additive manufacturing, surgeons can now create spinal cages tailored specifically for ...
VIEW MORE
Understanding Equivalence Testing Requirements for Proximal Lateral Tibia Plates Why Structural Identity Alone Fails Clinical Equivalence Validation Just because two plates have exactly the same size and screw hole placement doesn't mean they'll per...
VIEW MORE
The Clinical and Operational Necessity of SLAs for Ortho Surgical InstrumentsOR Workflow Dependencies: How Instrument Availability Directly Impacts First-Case-On-Time and Block UtilizationGetting the right surgical instruments ready on time is absolu...
VIEW MORE
Complaint Handling for Sternum Implant Malfunctions: Regulatory Compliance and Workflow FDA MDR Requirements for Sternum Implants: Reporting Timelines and Documentation Standards When it comes to sternum implant issues, medical device makers have no...
VIEW MORE
Sunshine Act Reporting Requirements in Specialty OrthopedicsWhat Must Orthopedic Device Manufacturers Disclose Under the Sunshine Act?Device makers in the orthopedic field have to track and report every payment or transfer of value over $1 they give ...
VIEW MORE
Why Returns and Loaner Sets Foot Ankle Instruments Demand Specialized Management Foot and ankle orthopedic surgeries need an incredible number of instruments - sometimes over 50 different tools for each operation - which creates all sorts of logist...
VIEW MORE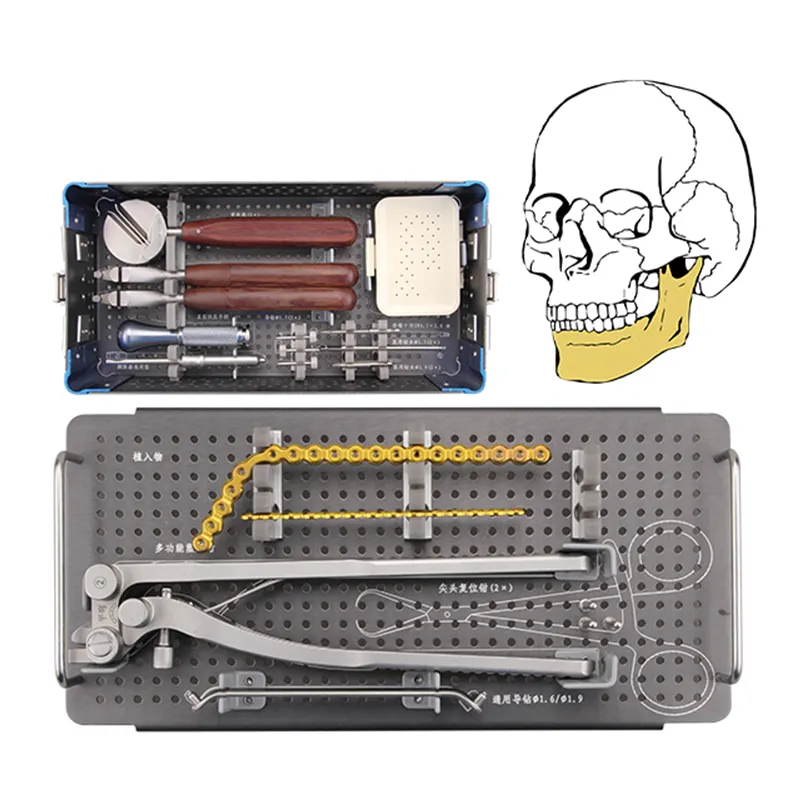
Strategic Negotiation of Distributor Agreements for Global Maxillofacial Sales Balancing exclusivity, territory scope, and market access in CMF device distribution Getting good distributor deals right for selling maxillofacial products worldwide mea...
VIEW MORE
Identify and Classify Core Trade Secrets in Titanium Mesh Co-DevelopmentDistinguishing proprietary titanium mesh IP from industry-standard know-howTo keep proprietary intellectual property safe, companies need good documentation showing what's truly ...
VIEW MORE
Regulatory Compliance and Risk Mitigation Through Early Cross-Functional Integration How siloed handoffs delay design transfer—and increase FDA review risk When R&D, manufacturing, and regulatory departments work in isolation, it creates serio...
VIEW MORE