Shuangyang Building, Yangshe Town, Zhangjiagang City, Jiangsu Province, China.
Shuangyang Building, Yangshe Town, Zhangjiagang City, Jiangsu Province, China.
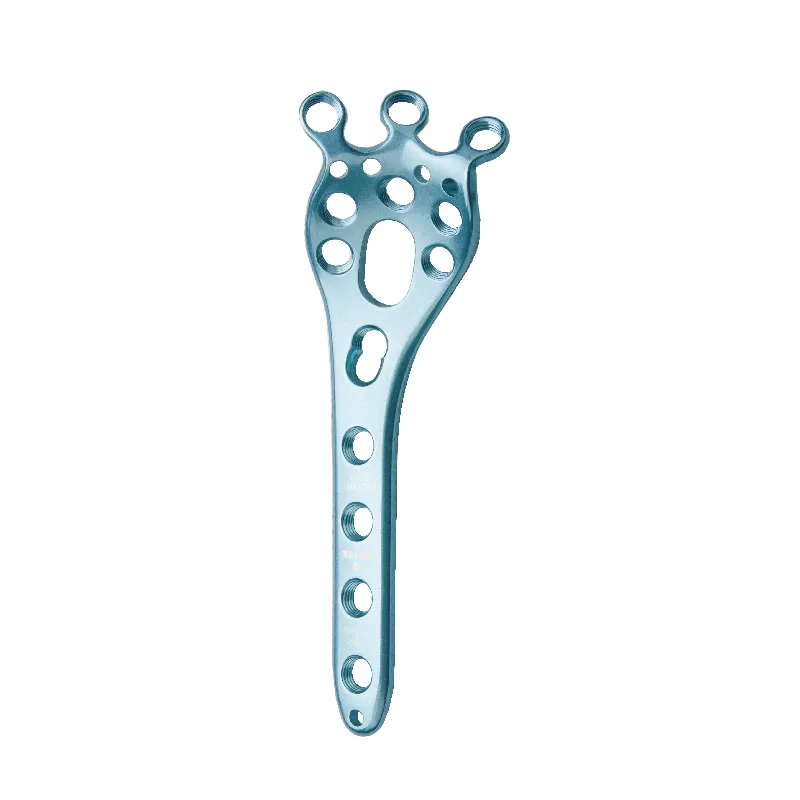
ASTM F382 as the Foundational Standard for Fibula Plate Mechanical Strength TestingScope, Applicability, and Why It Remains the Primary ASTM Standard for Metallic Bone Plates The ASTM F382 standard sets out how to test the mechanical properties of me...
VIEW MORE
Biomechanical Limits of Titanium Cable Knots in Sternal Fixation Shear stress failure at knot sites exceeding 350 N threshold When the shear stress on titanium cable knots goes over 350 Newtons at their yield point, they tend to fail in a pretty dra...
VIEW MORE
Why Finite Element Analysis Is Essential for Volar Locking Plate DesignThe application of Finite Element Analysis (FEA) has changed how we approach designing volar locking plates. Instead of relying solely on physical prototypes, engineers can now si...
VIEW MORE
Biomechanical Stability Testing: Core Requirements for Regulatory Submission Static and Dynamic Load Testing in Synthetic and Cadaveric Bone Models To properly validate the biomechanics of these coated proximal humerus plates, researchers need to co...
VIEW MORE
Regulatory Foundations for Validating Cleaning Instructions AAMI ST98, ISO 17664, and FDA Guidance: Core Requirements for Reusable Orthopedic Devices Getting cleaning instructions right for distal humerus plates means following those key regulatory...
VIEW MORE
Biomechanical Necessity of Torque Control in the Cervical Spine The cervical spine’s unique biomechanics—characterized by high mobility, variable bone quality, and proximity to critical neurovascular structures—demand precise torqu...
VIEW MORE
Why Surface Roughness Directly Governs Osseointegration in Titanium MeshBiomechanical Mechanism: How Microroughness (Ra 1.0–2.5 µm) Enhances Osteoblast Adhesion and Early Bone BondingTitanium mesh works best when its surface has a roughne...
VIEW MORE
The Unique Biomechanical Demands on Sternal Plates Cyclic Chest Wall Motion: Respiratory and Cardiac Loads Drive Repetitive Stress The sternum plates have to handle a lot of mechanical stress just from normal body functions. Breathing alone creates ...
VIEW MORE
Regulatory Foundations: How FDA Risk Classification Defines Class II vs Class III Cervical Spine Fusion Devices The Risk-Based Framework: Why Cervical Fusion Devices Fall into Class II or Class III Medical devices get grouped by the FDA according to...
VIEW MORE
Biomechanical Rationale: How Anatomical Contouring Improves Bone-Plate IntegrationReducing mechanical mismatch between plate geometry and complex craniofacial bone topographyMaxillofacial plates shaped to match facial bone contours tackle one of the ...
VIEW MORE
Radiographic Alignment: Foundational Key Performance Indicators for Anatomical ReductionRadial height, inclination, and volar tilt—evidence-based thresholds and clinical relevanceGetting accurate X-ray measurements is absolutely critical when e...
VIEW MORE
Regulatory Compliance as the Foundational Gatekeeper ASTM F67 and F136: Why Chemical Equivalence Alone Is Insufficient Getting materials to meet ASTM F67 standards for unalloyed titanium and F136 specs for Ti-6Al-4V ELI involves much more than just...
VIEW MORE