Shuangyang Building, Yangshe Town, Zhangjiagang City, Jiangsu Province, China.
Shuangyang Building, Yangshe Town, Zhangjiagang City, Jiangsu Province, China.
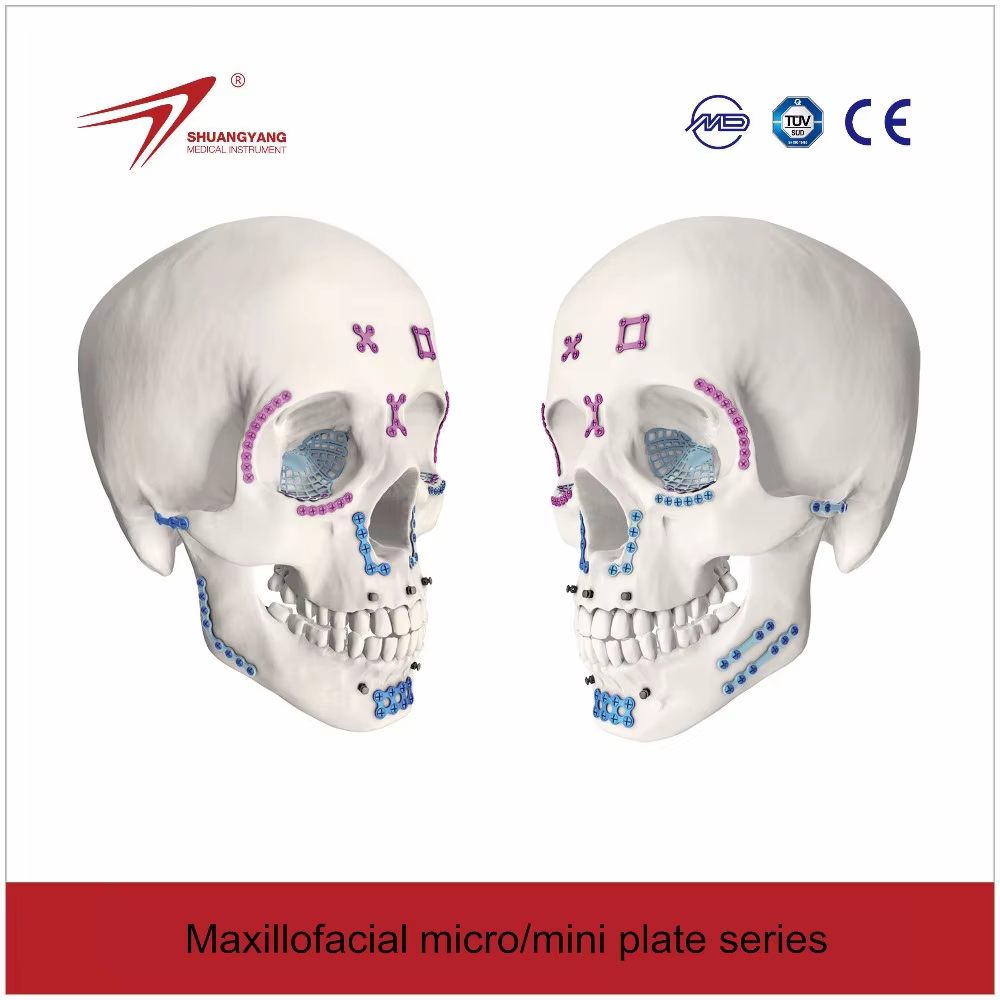
Strategic KOL Selection Tailored to Maxillofacial Trauma Practice RealitiesPrioritizing Oral & Maxillofacial Surgeons and High-Volume Trauma Fellows by Clinical Volume and Protocol InfluenceGetting key opinion leaders involved in maxillofacial trauma...
VIEW MORE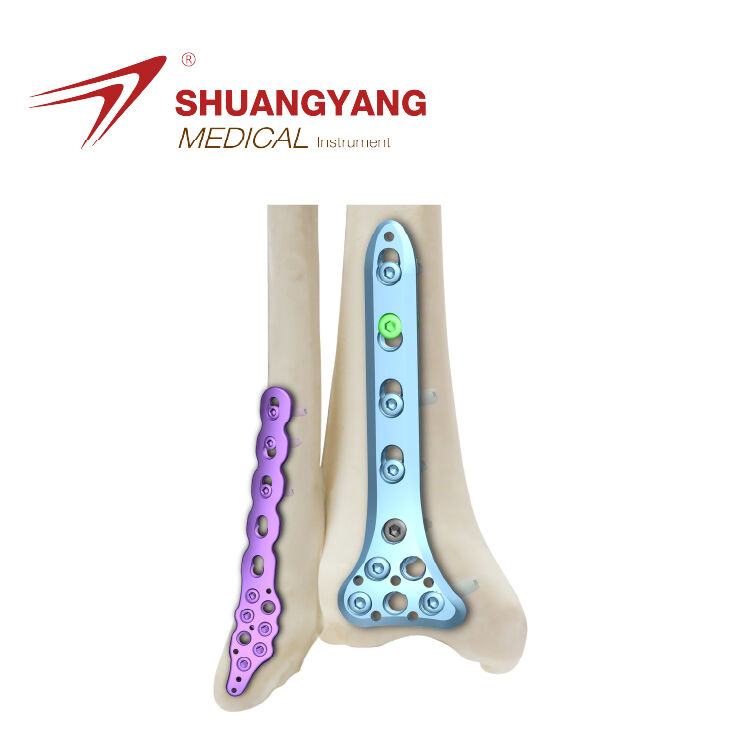
Define Key Benchmarking Criteria for Distal Humerus PlatesAnatomical Fit and Pre-Contoured Design AccuracyGetting the shape right matters a lot when fixing fractures in the lower part of the upper arm bone. Surgical plates need to match how the back ...
VIEW MORE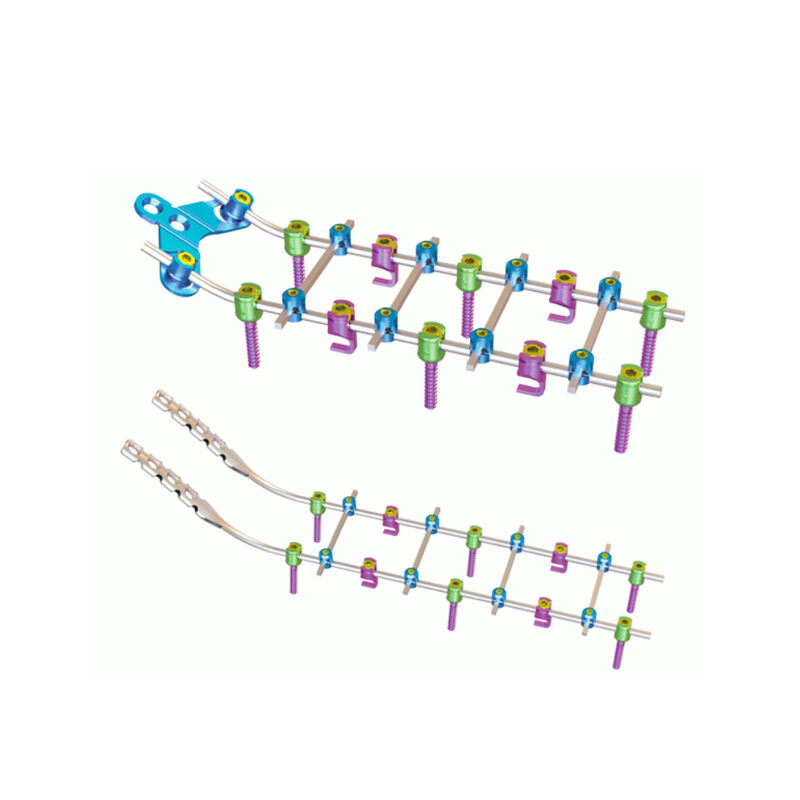
The Gatekeeper Role of Reimbursement Policies in Spinal Fusion Adoption The way money flows through healthcare acts as the main control point for how widely spinal fusion gets used across American medical facilities. Insurance companies and governme...
VIEW MORE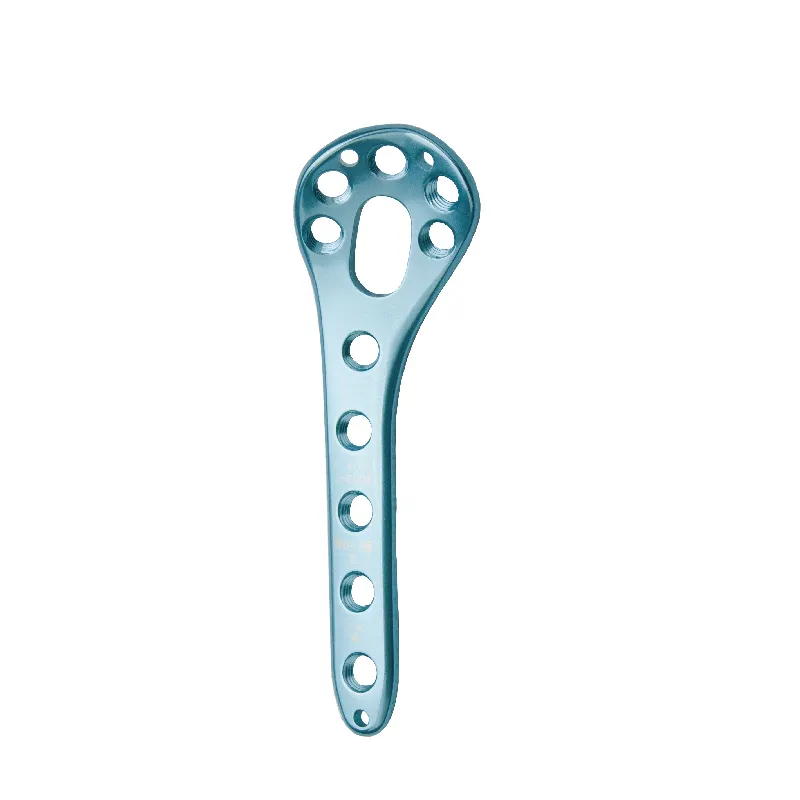
Understanding Unmet Clinical Needs in Pediatric Orthopedics Prevalence and consequences of untreated limb deformity, early-onset scoliosis, and pediatric trauma More than two thirds of pediatric orthopedic issues like limb deformities and early onse...
VIEW MORE
Regulatory Pathways and Market Access FoundationsFDA, CE Mark, and NMPA requirements for minimally invasive spine devicesGetting through the maze of regulations stands as the initial hurdle when bringing medical devices to market. For products enteri...
VIEW MORE
Why Emerging Markets Are Becoming Critical for Fibula Plate Adoption Surge in Orthopedic Trauma Burden Driven by Road Traffic Injuries and Aging Populations The demand for fibula plate solutions keeps rising in emerging markets because of two big pr...
VIEW MORE
Fragility Meets Function: Why Orbital Plates Demand Precision Shipping Microstructural Integrity as a Clinical Imperative Special care during shipping is absolutely necessary for orbital plates since tiny flaws can really impact how patients do afte...
VIEW MORE
ISO 11607: The Core Standard for Sterile Barrier SystemsPart 1: Design, Material Selection, and Sterilization Process CompatibilityWhen choosing materials for sterile barrier systems, compatibility with different sterilization techniques needs thorou...
VIEW MORE
Sterile Barrier Failures: The Leading Cause of Medical Device Packaging Recalls Seal Integrity Breakdowns and Loss of Sterility in Implant Pouches Sterile barrier systems act as the last line of defense against contamination for important medical de...
VIEW MORE
MRI Safety Fundamentals for Anterior Cervical Spine ImplantsDecoding ASTM F2503: Magnetic Resonance Conditional, Safe, and Unsafe ClassificationsWhen it comes to MRI safety for anterior cervical spine implants, everything starts with the ASTM F2503 s...
VIEW MORE
Why a 10^-6 Sterility Assurance Level Is Mandatory for Spinal Implants The Sterility Assurance Level, or SAL, basically tells us how likely it is that any living microbes will survive after sterilization. When we're talking about spinal implants spec...
VIEW MORE
Why Shelf Life Extension Is Critical for Titanium Cable Binding Systems As time passes, materials tend to break down which creates real problems for those titanium cable binding systems found in medical implants. Sure, titanium alloys are pretty good...
VIEW MORE